History of a disaster drug
History of a disaster drug
- 1956: Chemie Gruenenthal's drug Thalidomide is licensed in Germany as an anticonvulsant and anti-anxiety drug
- Also considered to reduce "morning sickness" in pregnant women it is sold over the counter in much of Europe
- 1961: Gruenenthal is presented with studies suggesting the drug's dangerous side-effects on unborn babies
- November 1961: Thalidomide withdrawn from sale
- 1973: UK distributor Distillers recognises and compensates 460 Thalidomide survivors
- Gruenenthal helps victims in 38 countries through two foundations it has set up, in recognition of its role in the health disaster.
Forgotten victims fight back
Avite was founded in 2004 by Jose Riquelme, who believes he only found out about his condition aged 17, when he came across a magazine in a rubbish dump near his home in Murcia in 1980 and read an article about Thalidomide survivors abroad.
His campaign group, which now has 286 members, successfully sued Gruenenthal in 2013, when a Spanish judge agreed that the company had not taken the necessary care to ensure that Thalidomide was safe.
The judge in that case ordered Gruenenthal to compensate 20 Spanish survivors out of the 186 named in Avite's lawsuit. But Gruenenthal won on appeal, arguing that after 50 years there could be no proof that these individuals' deformities were related to their drug.
 AVITE
AVITE
"No other substance can produce the malformations presented by 250 Spanish victims," according to Xavier Garcia Mora of the Catalan Association of Forensic Medics, who made a detailed study of Avite's members.
Dr Garcia Mora says 90% of those who suffered from the drug's side-effects would have died during childhood.
Drug company's role in Spain
Avite's campaigners have produced what appears to be a letter from Gruenenthal to its sister company in Madrid, dated 21 December 1961 - shortly after Thalidomide was withdrawn from European markets.
In the letter the German company accepts that it is not necessary to warn all Spanish doctors of the drug's danger due to "the extremely minor distribution" of Softenon, the commercial name used in Spain.
"Spain was like a Third-World country up to the 1980s," says Rafael Basterrechea. "Doctors were prescribing to illiterate people a lot of the time and pharmaceutical companies could do whatever they liked."
In a written response to the BBC, Gruenenthal says it "sincerely regrets the Thalidomide tragedy", but argues that distribution of Softenon in Spain ceased under company instructions in November 1961.
"However, in Spain, Thalidomide-containing medicines were also marketed by other companies which had (illegally) manufactured and marketed their own products independently [meaning] Gruenenthal therefore had no control over their operations."
Sofia Maria's story
Sofia Maria Garcia Duran, 46, spends the week at a care home in Sant Feliu de Llobregat, near Barcelona, and weekends with her mother Antonia and her husband.

She has never received any compensation for her condition, despite being born with no legs and tiny, fingerless stumps for arms.
"I could live alone if I could afford a flat in a decent condition to live in," says Ms Garcia Duran. "But all I get is €550 a month, and the care home costs €450."
Her mother was given unknown medication when suffering severe nausea during pregnancy in 1970.
Doctors who found out
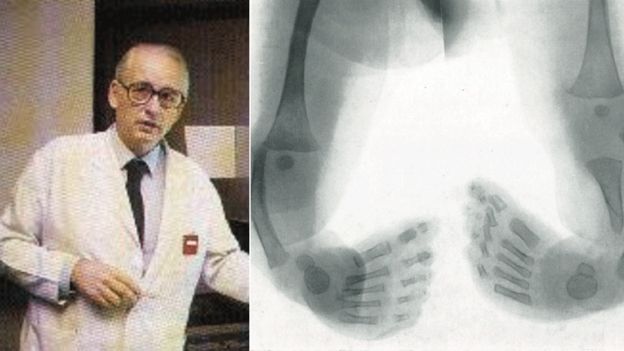
It was in Hamburg that Claus Knapp, 88, and senior partner Widukind Lenz uncovered the cause of so many deformities in babies.
"I was working as a radiologist, sitting there looking at plates all day. Then one day I saw the X-ray of a child with no bottom half: no hips, no legs, nothing.
"I set it to one side, as I had no idea what this was or what could cause this. Then a few more appeared. Every day I gave a demonstration to student doctors and hung up X-ray plates.
 CLAUSS KNAPP
CLAUSS KNAPP
"People said: 'Why are you always showing us this same case? We don't know what this is.' And I said: 'But it is not the same one - there are many'."
For three weeks in autumn 1961, the two partners drove around Hamburg visiting parents, many of them mourning a Thalidomide baby who had died, trying to find an explanation.
The common denominator was Thalidomide, taken by all of the mothers they had checked, from factory workers who helped themselves to freely available pills to help them get though their shifts, to others taken to hospital during their pregnancies for appendicitis or other ailments.
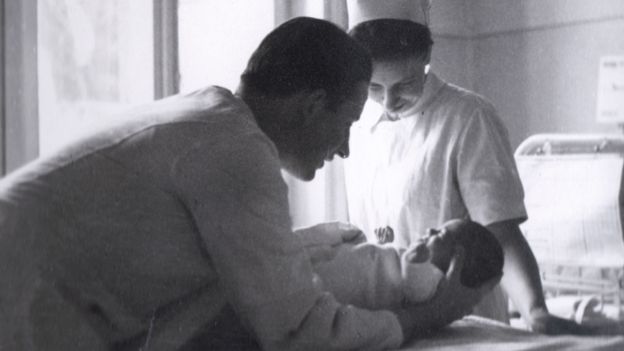 CLAUS KNAPP
CLAUS KNAPP
Dr Knapp moved back to his native Spain in 1963, and believed for many years that the country had been spared the scourge of Thalidomide, until he found out about Avite's campaign for justice.
Widukind Lenz has since died and Dr Knapp, shocked that the fight is still going on after 55 years, says "I'm the last witness".

Aucun commentaire: